Pricing and ordering details are determined by several factors. Please provide your contact information and we will be in touch shortly with more detailed product information.
Get in Touch
The OSOM® COVID-19 Antigen Rapid Test is a lateral flow chromatographic immunoassay intended for the qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen in direct mid-turbinate (MT) nasal swab specimens collected by a healthcare provider from individuals suspected of COVID-19 within the first 7 days of symptom onset.
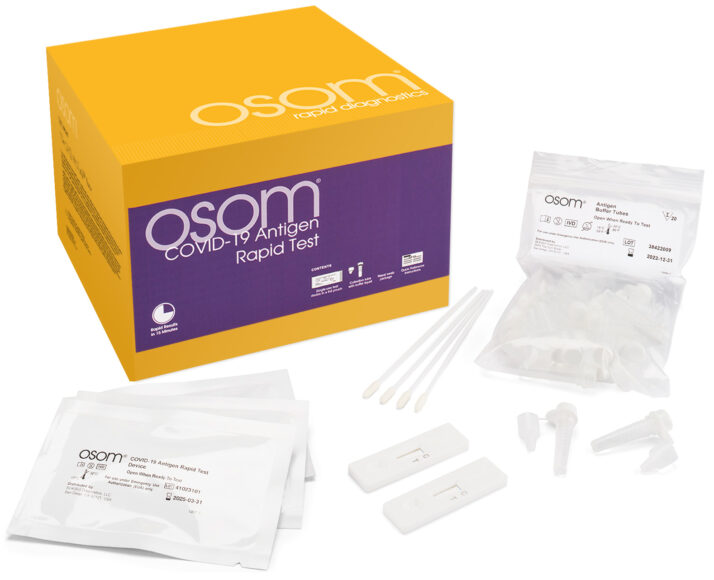
This test has not been FDA cleared or approved. It is authorized by FDA under an EUA for use by authorized laboratories. It has been authorized only for the detection of SARS-CoV-2 antigen, not for any other viruses or pathogens and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C S360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
USA - Not available in all countries. Contact us for availability.
Mid-Turbinate Swab
Pricing and ordering details are determined by several factors. Please provide your contact information and we will be in touch shortly with more detailed product information.
Get in Touch